Clinical and commercial
Working with partners to adopt our technology and reach more underserved communities

A breast exam with Bexa accurately identifies abnormal breast tissue, even under the arms, in minutes, with no discomfort or radiation.
Bexa is a Class II FDA cleared (K181672) Class II medical device that is currently indicated for the documentation abnormal breast tissues and masses discovered on a breast examination.
A breast examination with Bexa is quick, painless, involves no radiation and provides women with an immediate result.
Multiple U.S. clinical studies either completed or nearing completion define the power of a breast exam with Bexa to discover abnormal masses, and to drive social change and economic impact that result from women’s consistently very high adoption of breast exams with Bexa.

Stop failing women
It’s time that we open our collective eyes to the realities of most breast cancer screening programs: they fail to engage the majority of women.
Breast cancer screening programs that rely solely upon one the widespread availability of a single, poorly adopted modality, fail women. Without widespread availability and adoption, there’s no significant impact upon the health of populations.
To be very clear: mammograms have saved hundreds of thousands of lives and we are in no way ‘anti-mammogram.’ Instead, we are ‘pro-reality,’ and that reality is that the adoption of mammography by women in most countries is very poor.
It’s time for and additional modality, one that can be made easily available and that women will adopt. That modality is Bexa.
Bexa in numbers
approximate 5-year mortality with undetected/untreated DCIS – stage 0
approximate 5-year mortality with unscreened but treated invasive breast cancer – stages I-IV
percentage of women enthusiastic about Bexa
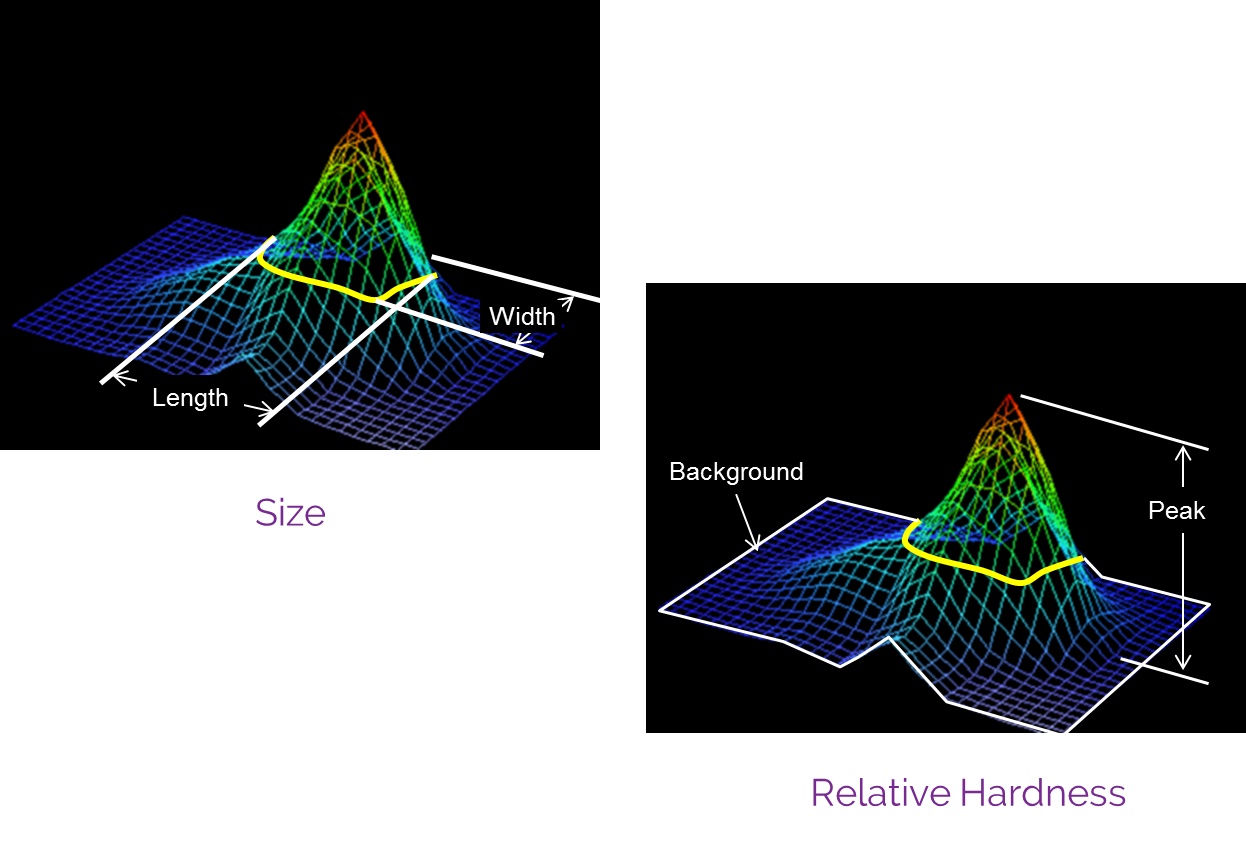
How it works
A breast examination with Bexa fully integrates the evaluation of all discovered breast masses using B-mode ultrasound, adding critical data to the evaluation and classification of abnormal breast tissues, and dramatically reducing an already low need for additional imaging studies.
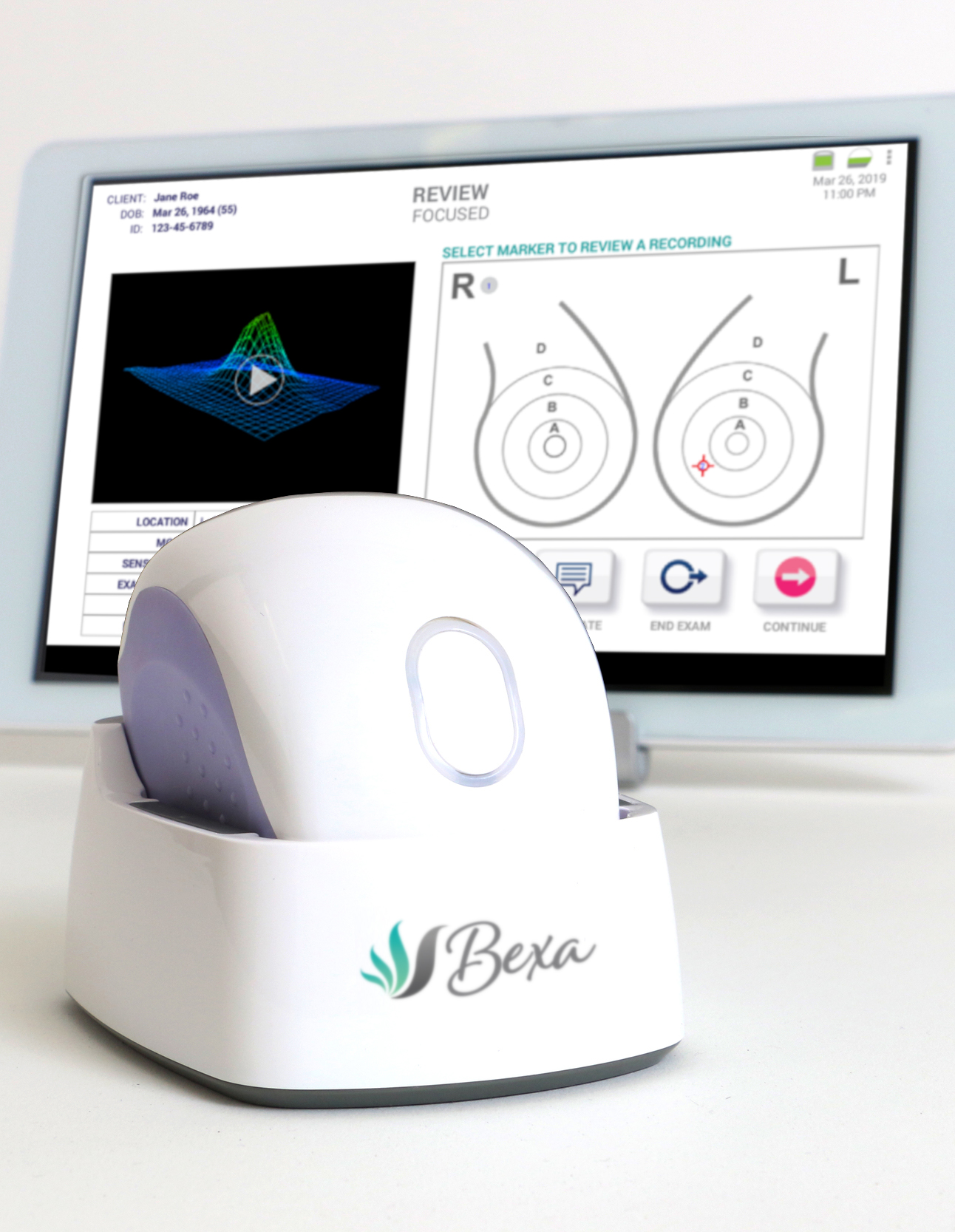
Bexa is an opportunity
Even low-cost Bexa examinations represent new and profitable revenues for your practice, facility or system.
Most of the ‘marketplace of women at risk,’ and the opportunity they represent, is lost to physicians who only offer historic technologies.
Bexa represents an opportunity to ‘do good,’ while adding a new service line and new business.
Thank you for taking the time to learn about Bexa
We’re working hard to continuously advance the sophistication of Bexa and to establish its role in the early detection of breast cancer.